TL/DR: Here’s what’s new in reproductive health research as of October 2020: Researchers have found that estrogen and progesterone may be protective against COVID-19; the diabetic drug metformin may be helpful for PCOS patients struggling with infertility; a new treatment may offer relief for uterine fibroids.
Here at Flex®, we’re constantly checking in with our team of reproductive health experts and OB-GYNs to find out what’s new on the medical scene – asking about everything from recently published research to cutting-edge treatments and therapies for conditions like endometriosis, uterine fibroids, PCOS, and infertility. We think it’s so important to keep our staff and our customers up-to-date on critical developments, especially the ones that have the potential to majorly impact our lives.
That’s why we’re excited to introduce a new blog series, “Updates in Reproductive Health Research,” to give our readers a quick monthly roundup of the news, stats, and advancements impacting the industry right now. This month’s update, compiled by MD-MPH candidate and Flex medical reviewer Jahnavi Curlin, covers some interesting hormone-related developments in COVID-19 research, a newly approved therapy for uterine fibroids, and findings re: a drug that may help PCOS patients trying to get pregnant via IVF. Let’s dive in:
A hormone-based treatment approach to COVID-19
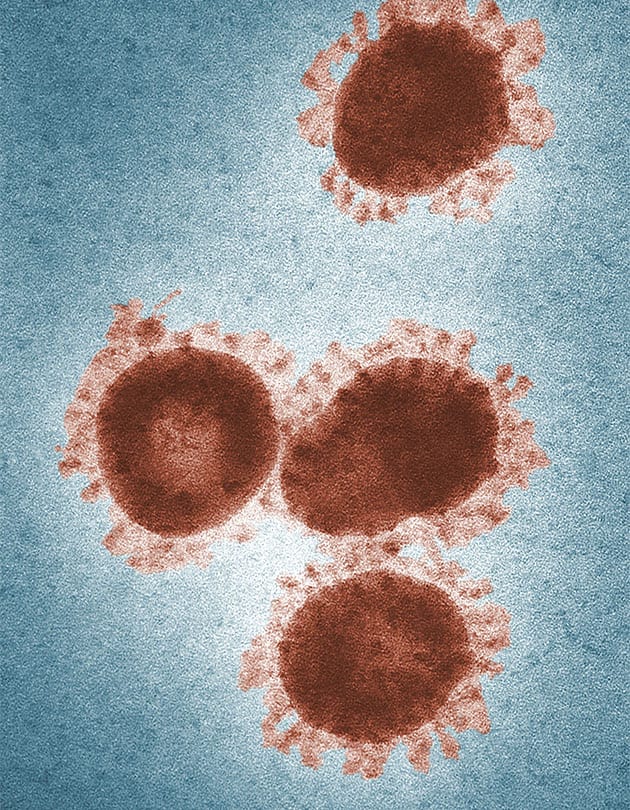
This month’s edition of industry updates couldn’t begin without addressing the elephant in the room: COVID-19. As a brief reminder (in case you haven’t been glued to your phone for the last seven months like we have), COVID-19 is the disease caused by the novel coronavirus, SARS-COV-2. As of October 1, the disease has infected more than 35 million and led to the death of 1.04 million people around the globe.
While pharmaceutical companies are scrambling to launch an effective vaccine by early 2021, there’s still much debate around when such a vaccine could become widely available. Meanwhile, thousands of other medical researchers have been working on a treatment for patients already infected with the virus: While no treatment has been officially released, the FDA has (as recently as August 23rd) approved several therapies, including the drug remdesivir for emergency use in advanced cases.1
However, there is another potentially viable treatment being tossed around in scientific circles that’s made it as far as two clinical trials (now taking place in New York). This treatment may sound familiar to you, Flex readers: it’s none other than the pair of hormones that drive your monthly cycle, estrogen and progesterone.
While we’re still learning more about this virus in real-time, researchers at Tulane found that COVID-19 patients assigned female at birth had less severe disease and better recovery outcomes than those assigned male.2 Why? Because estrogen and progesterone have been found to calm down COVID-19’s damaging “cytokine storm.”
The long and the short of what we know is that estradiol (a form of estrogen) and progesterone have hardcore anti-inflammatory properties. One of the things that they can do is reduce the number of interleukins in your bloodstream. Interleukins are basically the “first responders” of your immune system, and interleukin-6 (IL-6), in particular, is one of the major components of the cytokine storm that makes COVID-19 so deadly.
Researchers are continuing to study the potential benefit of estradiol and progesterone hormone therapy on COVID-19 patients. One benefit to this treatment approach is the wealth of knowledge scientists already have about the pharmacology, dosages, and potential toxicity of hormone therapy – making it easier to speed up off-label use in potentially life-threatening cases of the virus.2 We’ll continue to keep an eye on the research and provide an update should this treatment be approved by the FDA.
A new option for individuals with PCOS undergoing fertility challenges
In other news, for individuals diagnosed with polycystic ovary syndrome (PCOS), new research suggests that the drug metformin may be helpful for IVF-assisted pregnancy for certain groups of people. Some background: PCOS is a hormonal disease that tends to cause infrequent or absent menstrual cycles. When those with PCOS do get a period, it may be especially painful or heavy – and show up totally unannounced.
Periods aren’t the only challenge faced by individuals with PCOS (though we hope to have made them a little bit easier with the Flex Disc™, which has been shown to reduce cramps and can be worn for longer periods of time than traditional menstrual products, even on heavy days). Many people with PCOS have difficulty becoming pregnant because they ovulate irregularly. As a result, many turn to therapies such as in-vitro fertilization (IVF).
A recent study found that individuals with PCOS who took metformin while pursuing IVF had an overall decrease in something called ovarian hyperstimulation syndrome, which is a painful and potentially harmful condition that happens when taking the extra hormones during IVF.3 And, interestingly enough, the group of individuals given metformin (and who had a BMI greater than 26) was associated with a significantly greater clinical pregnancy rate (e.g., more women became pregnant while on the drug).
For reference, metformin is an oral medication that is often prescribed for diabetes. It works by decreasing glucose production while simultaneously increasing the amount of glucose used by the body, netting out to a lower overall glucose or blood sugar level.
The science behind why a medication designed for diabetes seems to improve fertility for PCOS patients undergoing IVF is complex – these findings do, however, have the potential to significantly impact the fertility prognosis for individuals with PCOS. It’s something to consider asking your OB-GYN or healthcare provider about, especially if you match these criteria: Diagnosed with PCOS, BMI of 26 or higher, and seeking pregnancy through IVF.
Oriahnn for uterine fibroids
Finally, a promising new treatment for uterine fibroids was recently approved by the FDA.
FYI, uterine fibroids are non-cancerous growths that develop in the uterus, often leading to symptoms like pain in your nether regions or long, heavy periods. While many Flex users with fibroids have had a lot of success dealing with their periods by switching to Flex Disc or the Flex Cup™ (the Slim Fit holds the equivalent of 2 super tampons; the Full Fit holds 3 super tampons’ worth of period blood), heavy bleeding can still be a major pain, getting in the way of day-to-day activities.
If you’ve been searching for another way to manage bleeding caused by uterine fibroids, you may be in luck: A new drug called Oriahnn was just approved by the FDA.4 It’s a combination product containing one pill with elagolix, estradiol, and norethindrone acetate (taken in the morning) and another pill with only elagolix (taken in the evening). As a whole, Oriahnn can be taken for up to 2 years.
Clinical studies showed that the majority of patients (68.5% in the first study, 76.5% in the second study) on this drug had a 50% decrease in menstrual blood loss at the end of the study compared to the beginning. In addition, participants’ final menstrual period during the study had blood loss of less than 80 mL (where 80 mL and above is the clinical definition of a heavy menstrual period).
To summarize this long list of numbers, the drug was associated with a lighter menstrual flow for women in both clinical trials – a finding that could have an incredible impact on quality of life for women with uterine fibroids. Oriahnn joins the list of other, existing treatments for uterine fibroids, including hormonal birth control and surgery.
This article is informational only and is not offered as medical advice, nor does it substitute for a consultation with your physician. If you have any gynecological/medical concerns or conditions, please consult your physician.
© 2021 The Flex Company. All Rights Reserved.