Briefing you on the latest in reproductive health research
This month’s issue of industry updates in period and reproductive health research covers an array of topics. We’ll take a look at:
- Research on the link between contraception and pleasure
- Some newly released data on possible treatments for women with the BRCA mutation
- An immunotherapy option for endometrial cancer that just landed on the FDA accelerated track
Does contraception increase sexual pleasure? The data shows…
Here at Flex®, we’re always eager to discuss contraceptive methods and the ways in which they add to our complete sexual lives (yes, you still need birth control when having period sex!). Unfortunately, the experience of taking or using birth control hasn’t historically been emphasized in medical research—especially with respect to female pleasure and sexual wellness.
This month, we’re covering a study that’s taking steps to change this: Titled the “HER Salt Lake Contraceptive Initiative,” the study was conducted jointly between the University of Wisconsin at Madison and the University of Utah and was built on a cohort of over 2,000 women.1
Each study participant received family planning and contraceptive services at the outset. Participants were counseled on a variety of contraceptive options and, once they decided on their preferred method, were followed up with at the one-, three-, and six-month marks.
At each milestone, participants were evaluated in multiple ways: They answered questions ranging from the function of the BC method itself to their sexual happiness and wellness, and were also evaluated for potential bodily changes (like breast tenderness, mood changes, or breakthrough bleeding).
The group as a whole chose a healthy mix of contraceptive methods, including the hormonal and non-hormonal IUDs, the vaginal ring, “the shot” (an injectable form of BC that is given every three months), the arm implant (as made popular by Vanessa Hudgens), and “the pill” – a.k.a. combined oral contraceptives (COCs).
Here’s what they uncovered:
- At the one-month mark, 52.8% of participants cited that their new method improved their sex life (26.1% “improved a lot” vs 26.7% “improved a little”); 16.8% in total said it made their sex life worse, and 30.4% reported no effect on their sex life
- Of note, those folks who said that their contraceptive made their sex life worse had higher odds of discontinuation (3.3x that of their counterparts by the six-month mark). Common reasons that participants chose to discontinue their BC method of choice? Excessive bleeding, increase in PMS-type symptoms, and mood-related side effects were all frequently cited.
- The factor most commonly found to influence the decision to stop using birth control? Changes related to bleeding (i.e. spotting, heavy bleeding, or irregular bleeding)
So, what did researchers make of the group that reported a positive change in their sex lives? A few reasons stood out, ranging from logical—for instance, feeling more at ease because of the pregnancy protection or being able to have sex more frequently thanks to a more regular or lighter period—to subtler factors, like sexual spontaneity.
Basically, for those who found birth control serendipity (minimal negative side effects, plenty of positive changes), their sex lives improved—at least to a small degree.
But don’t forget the other side of the coin: Not every type of contraceptive works the same way for everyone, and, as these researchers confirmed, it can take a bit of Goldilocks action to find the one that’s right for you.
While we can’t yet say that one specific type of birth control is linked to increased sexual pleasure or desire, it’s exciting to at least have this research out there. It’s a step in the right direction, affirming contraception as an important area of study—especially with regards to side effects and impact on sexual function and wellness.
Breast cancer research shows favorable results for BCT
On another note, genetic testing has entered the healthcare space…and it’s brought up almost as many questions as it has answers.
Remember when Angelina Jolie made waves in 2013 when she penned an op-ed sharing her deliberation on choosing to pursue a mastectomy? That all started with her receiving the news that she had the BRCA1 gene.
Well, BRCA1 is still a hot topic today: It’s one of the genes that’s been implicated in increased risk of breast and ovarian cancer. It’s a tumor suppressor gene, which means that, when it’s missing (or mutated), the risk of unrestricted cell growth–and cancer–goes up.
It used to be that genetic testing required expensive medical procedures. Nowadays, however, there are a plethora of consumer-facing companies that aim to bridge the gap in accessibility and make genetic testing more widely available. This then brings up the question…what do we do with all this newfound knowledge? And for those who do test positive for the mutation, how are they addressing it?
Mastectomy, a.k.a. the removal of one or both breasts, is one option in such cases. Another option is breast-conserving therapy (BCT) with radiotherapy. These two modalities can also be combined, meaning that you go through radiotherapy prior to surgery, or the other way around. Developing such regimens requires access to a lot of data (and research plays a major role, too).
Now that more people are getting tested for the BRCA mutation, larger-scale studies are finally possible. One such study just came out of the Peking University Cancer Hospital and Institute in Beijing, China.2 Researchers there developed a cohort of women with BRCA1 or BRCA2 mutations and conducted a retrospective study—they looked backwards to see what type of treatment each person ultimately underwent as well as the large-scale outcomes in each population segment.
Although past research has compared BCT to mastectomy in general, this study took things a step further by focusing specifically on women with the BRCA gene mutations to see if outcomes changed. The main outcome studied was survival, and they also looked at rates of recurrence, or return of cancer.
After making adjustments, researchers found that those who underwent radiotherapy had comparable survival rates to those that underwent mastectomy surgery in the BRCA1/2 variant carriers. This is significant because it tells us that BCT plus radiotherapy may be a viable option for the subset of folks who would rather conserve their breasts while undergoing treatment.
As always, each individual circumstance is unique. Clinical expertise is key when coming up with the right plan of attack (whether preemptively or post-diagnosis) for a BRCA mutation. Hopefully, even more large-scale research will be carried out in the near future, moving the conversation forward for patients and providers alike.
Fast track for endometrial cancer immunotherapy
Speaking of genetics, the FDA has offered accelerated approval to a monoclonal antibodyMonoclonal antibodies are blood proteins made in a lab that mimic your natural antibodies (the things that help you fight off foreign invaders like viruses, fungi, and bacteria). These antibodies can then go on to do many things, from stopping cell growth to blocking viral particles from entering your cells. drug that treats endometrial cancer (!) with an emphasis on cancer that has a specific genetic feature called dMRR.3
As a reminder, the endometrium is the lining of your uterus. And as with many other parts of the reproductive system, endometrial tissue is capable of hosting both pre-cancerous and, eventually, malignant (cancerous) cells. Endometrial cancer is usually treated with surgery or platinum-based chemotherapy.
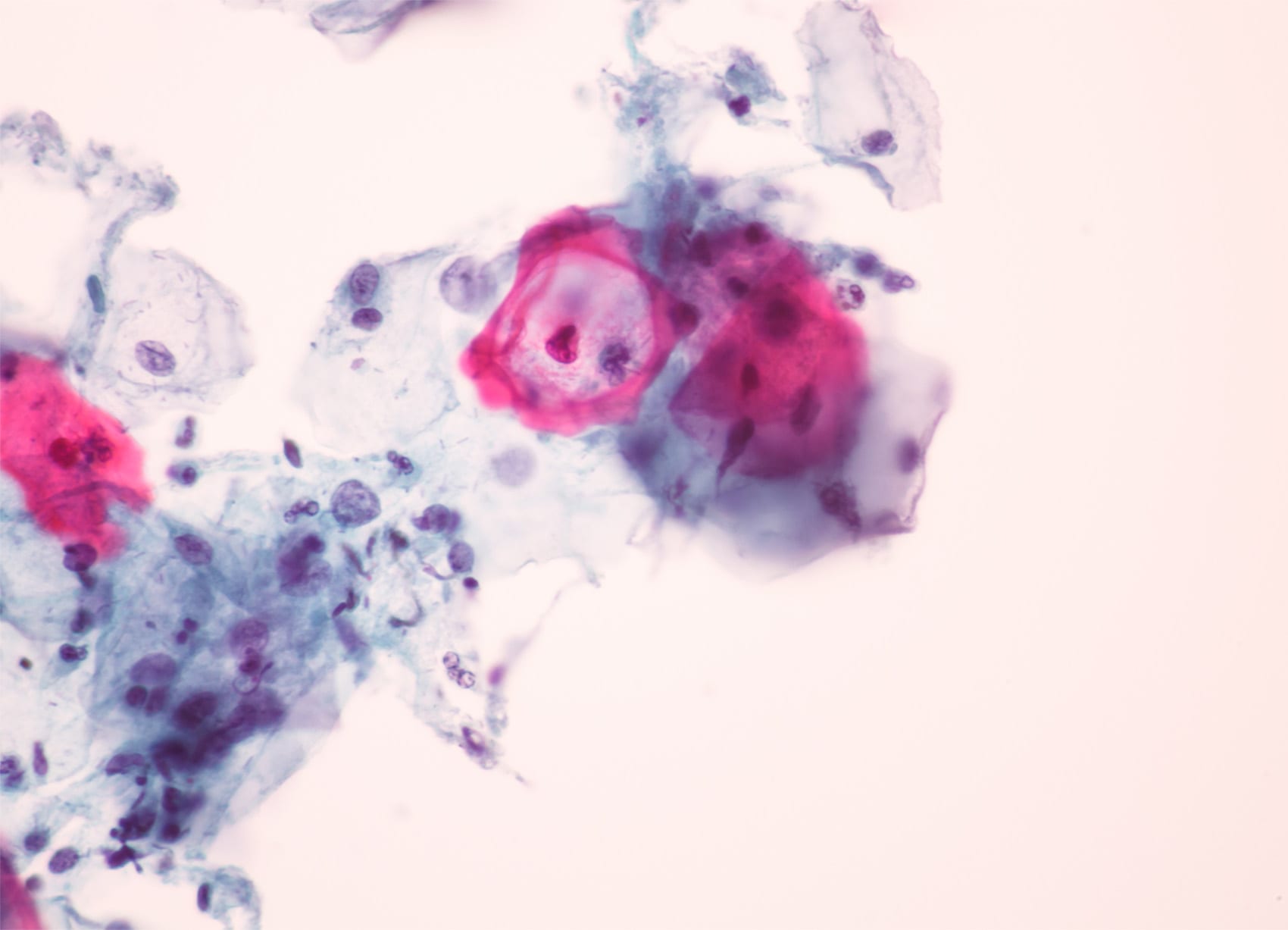
Quick side note: That Pap smear test you get every few years at the gyno (usually every three years, but it may be five years if taken with a HPV blood test) tests *somewhat* for endometrial cancer in addition to cervical cancer.
A Pap isn’t as sensitive or specific to the endometrium, though, so the best bet is usually an endometrial biopsy: Your clinician will take a tiny piece of the uterine lining and submit it to a lab for staining; this is what confirms the diagnosis. This is also where genetic screening is done to tell what subtype of cancer you’re dealing with and, therefore, which therapy is appropriate.
Although a majority of cases of endometrial cancer can be resolved with some form of surgery or chemotherapy, there are a handful of cases that do not respond to that standard of care—and it is for those cases that new and different immunotherapies are in varying stages of development. These immunotherapies use cellular pathways to block development of cancerous cells upstream. They’re often infused, meaning they’re given at regular intervals through an IV.
In the case of this particular drug, called dostarlimab or Jemperli, 42.3% of patients saw a complete or partial response (defined as tumor shrinkage) in their clinical study. Moreover, 93% had a response that lasted for at least six months. This is exciting news and could reflect an alternative treatment for people with genetically rare endometrial cancer. We’re hopeful for a speedy approval by the FDA.
That concludes this round of April industry updates! Tune in next month for more and check out our previously published editions below.
This article is informational only and is not offered as medical advice, nor does it substitute for a consultation with your physician. If you have any gynecological/medical concerns or conditions, please consult your physician.
© 2021 The Flex Company. All Rights Reserved.